Vaccine for cervical cancer arrives on mainland after approval by authorities

Initial shipments of Cervarix, the first vaccine for cervical cancer to be approved for use on the Chinese mainland, has passed inspection by Chinese quality authorities and is heading to health clinics across the country, pharmaceutical company GlaxoSmithKline said on Monday.
Cervarix, developed by GSK, was approved by the China Food and Drug Administration in July last year. Imported from Belgium, the first batch of nearly 275,000 doses of vaccine arrived in China, beginning in Chongqing and Nanchang, Jiangxi province, said Susan Song, a communications manager at GSK China.
The vaccine will meet the needs of a great number of Chinese women, the company said in a statement.
"Like other vaccines, Cervarix can be administered at community hospitals and health service centers," it said.
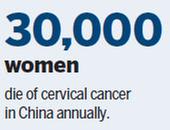
There are about 100,000 new cases of cervical cancer in China annually, causing more than 30,000 deaths every year, according to Qiao Youlin, head of the Epidemiology Department at the Cancer Hospital, Chinese Academy of Medical Sciences, in Beijing.
"Cervical cancer is the third-most common cancer among women between 15 and 44," he said. "Cervical cancer vaccination together with cervical cancer screening will significantly reduce the incidence."
Thomas Willemsen, general manager of GSK China Pharmaceuticals and Vaccines, said the company is undertaking a series of initiatives, including public disease awareness education and training to physicians on using the vaccine to enable more Chinese females to benefit.
Cervarix has been registered in 132 countries and regions, and more than 69 million doses have been administered worldwide, according to GSK.
Zhao Fanghui, a professor specializing in the prevention and treatment of cervical cancer at the Chinese Academy of Medical Sciences, said the vaccine has been in use for more than 10 years and proved to be reliable and effective, especially among adolescent girls from 9 to 15 years old.
"Human papillomavirus, which causes cervical cancer, is transmitted through sex," she said. "In many countries, it is most recommended to women between 9 and 15 years old, as the vaccine produces the best results on them, but it can also benefit older women."
A clinical trial in China, backed by CFDA and covering more than 6,000 females between 18 and 25 from 2008 to 2015, showed the vaccine generally to be more than 90 percent effective, she said.
Because of its high cost, the vaccine may not be included in China's national immunization plan. The three doses required for the vaccination may cost more than 1,700 yuan ($253) in China, Zhao said.
Some Chinese companies are also researching vaccines for cervical cancer, and some are already the subject of clinical trials, she said.
It is expected that domestically made vaccines will be available at lower prices in the next few years, and eventually they may be included in the national vaccine program, Zhao said.
- China's CR450: A new era of high-speed rail at 400 km/h
- TAN SUO SAN HAO to pioneer future of deep-sea exploration
- Xi's discourses on Chinese modernization published in Japanese
- Officials summoned over alleged garbage bin food served to students
- Caring hearts help to enhance quality special education
- Xi sends condolences to South Korean acting president over plane crash